This year’s Helsinki Chemicals Forum (HCF) took place virtually on the 27th and 28th of April. Ecobio also joined the Forum as probably many of you too. In this brief blog we would like to share with you what we got out of the lively discussions during the two days. In these take-home messages, we concentrate in the EU’s Chemicals Strategy for Sustainability. This is because it was by far the most heavily debated topic at the HCF.
As we all know, the EU’s new growth strategy, the European Green Deal, has set the European Union (EU) to become a sustainable climate neutral and circular economy by 2050. Therefore, it sets the goals to tackle pollution and move towards a toxic-free environment. The EU’s Chemicals Strategy for Sustainability (CSS), published in October 2020, is part of this scope. Not only because the chemical manufacturing industry is the fourth largest sector in the EU, but also because the chemicals are used in 95% of all manufactured goods.
Safe and sustainable-by-design to protect human health and the environment
The future chemicals have to be safe and sustainable-by-design, the CSS outlines. Although the actual meaning of this is yet to be defined in the EU, the debate during the HCF was around the following lines. New green chemistries need to be developed and used to produce new types of molecules to replace the most harmful current ones. The safe and sustainable-by-design concept needs to go through the entire life cycle of the chemical. For example, at the sourcing stage of the raw materials, the workers’ safety and human rights need to be adhered, and similarly at the manufacturing stage, too. The manufacturers also need to produce their chemicals, materials, and products environmentally friendly, e.g., by using renewable energy sources.
The safe use of the chemicals, materials and products must be guaranteed. In the final stage of their life cycle the waste must be recycled in a manner that contributes to the circular economy. Information and transparency from the start to the end of product’s life are the key. Currently, we do not know anything about 70% of the chemicals, which was reminded at the HCF. Some speakers pointed out that without the right information through the entire product-chain we would not know how to appropriately recycle the waste in the end, in particular of the long-lasting materials such as concrete. As a starting point to improve this and to enable consumers to make informed choices, ECHA is going to make the data in its SCIP-database publicly available by the end of this year.
Innovation and funding are prerequisite for the new developments
The CSS promotes innovation to develop the new chemicals of safe and sustainable-by-design. It was made clear during the HCF discussions that funding is needed at all fronts from the development to manufacturing until the waste management. The EU will provide funding for these innovations; they could be e.g., new green chemistries, new greener technologies at the manufacturing sites or novel ways to decontaminate waste.
Voices were heard at the HCF that funding is highly important. Concerns were raised how competitive the EU’s future chemicals sector would be if the non-EU-economies do not follow similar green strategies. It was also pointed out that chemicals sector will face many challenges simultaneously between now and 2050: green and digital transition challenge, circularity challenge and CSS. The CSS is a bigger regulatory update than REACH ever was, some HCF speakers noted. It was acknowledged, however, that the CSS gives a great opportunity for the EU’s chemical industry to be a global front-runner.
Essential use of the chemicals is the way forward
The CSS brings forward the concept on the essential use of the chemicals. Again, this is yet to be defined by the EU. Nevertheless, it was discussed in the HCF that in the evaluation process of the chemicals, the essentiality of their uses should also be assessed. The CSS outlines that the essential use must be a justified use where the most harmful chemicals are only allowed if their uses are necessary for health, safety or are critical for the functioning of society, and if there are no alternatives. Examples were given from the medical device sector; while the same harmful chemical used in a medical device could be considered essential, in the consumer products it should be banned.
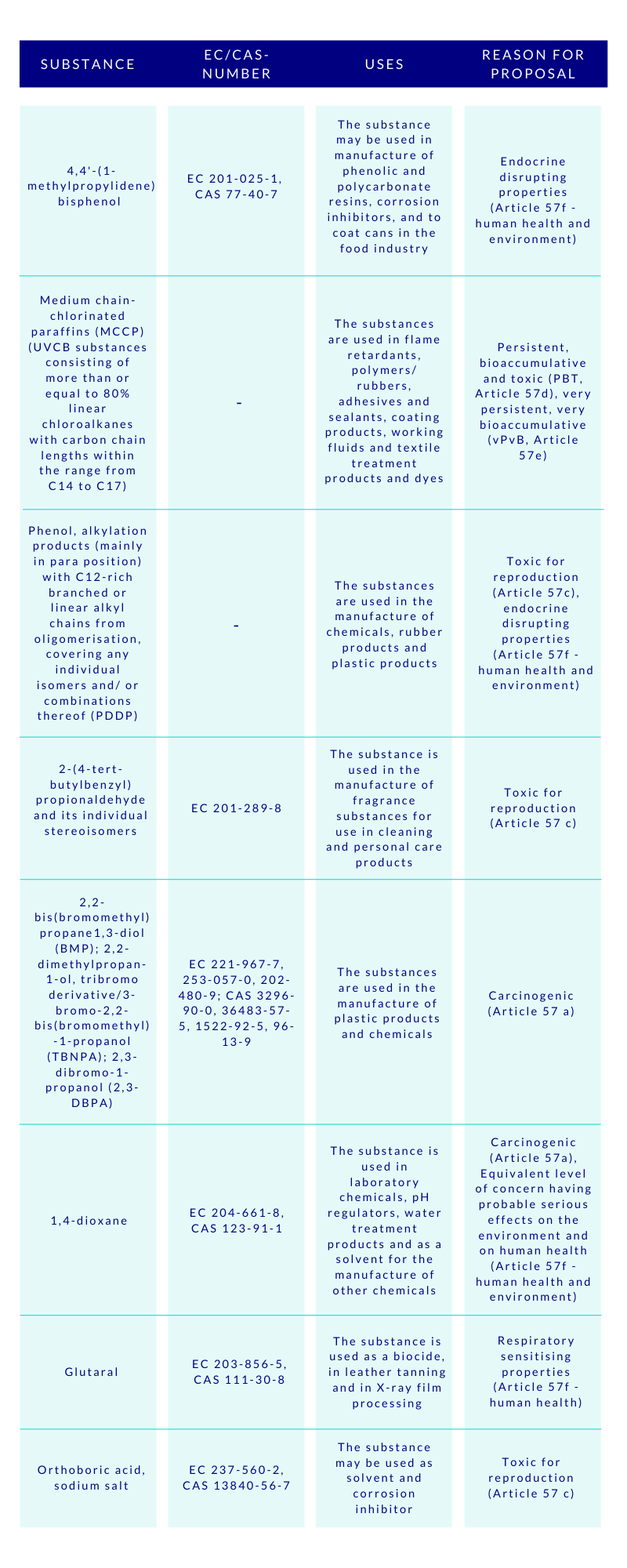
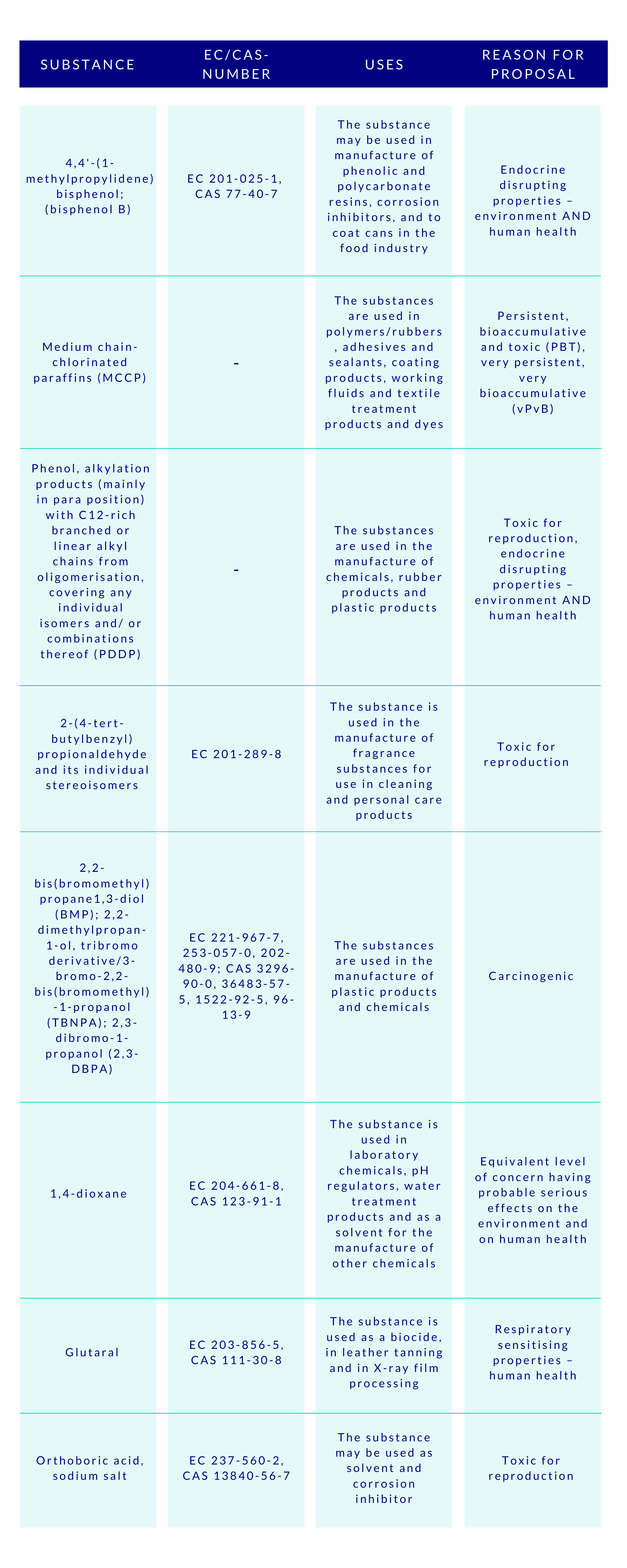
The CSS clearly aims at ensuring with the generic risk management approach that consumer products such as food contact materials, toys, childcare articles, cosmetics, detergents, furniture, and textiles, do not contain chemicals that cause cancers, gene mutations, affect the reproductive or the endocrine system, or are persistent and bioaccumulative. To empower this, the substances will be assessed and regulated in groups, instead of one-by-one. This will speed up the assessments made by ECHA, and consequently increase the number of restricted substances. Concerns were raised by some HCF speakers, whether this approach to regulate chemicals in groups would lead to omission of the essential uses of some specific chemicals. Time will tell.
PFAS and endocrine disrupters in the spotlight
Special attention is given by the CSS to PFAS and endocrine disruptors (EDs). The aim is to ban the use of PFAS as a group in the EU, unless proven essential for the society. As regards to the EDs, their all non-essential uses will be banned in the consumer products. The discussions are ongoing to introduce a new hazard class on endocrine disruptors in the CLP-regulation, based on the WHO definition, but building on the present criteria currently applied to pesticides and biocides. At the HCF, it was asked whether this new class would effectively be better to be added to the UN’s GHS to avoid the differences.
REACH will be re-opened in 2022
To allow the regulatory changes described by the CSS, the REACH-regulation will be revisited and amended accordingly. Information requirements are expected to increase. The CSS points to the direction to extend the REACH scope to cover certain polymers of concern, such as with CMR or ED properties. Also, information on the overall environmental footprint of the chemicals (e.g., emissions of greenhouse gases) would be required and more information will be needed to enable effective identification of the critical hazards of the substances (e.g., neurological effects).
The future REACH will also require data to enable identification of all carcinogenic substances manufactured or imported into the EU irrespective of their volumes. Furthermore, compliance of all REACH-registration dossiers is required. This is to strengthen the principles of “no data, no market” and the “polluter-pays”. In case of non-compliance, the registration numbers will be revoked. Mixtures of the chemicals will also be introduced in the updated REACH, considering also other relevant legislation. E.g., food additives, food contact materials, water, cosmetics, and detergents. To involve the other regulatory sectors better, one-substance-one-assessment approach will be employed by building the new assessment on the previous assessments of the substance. Finally, the ongoing discussions on the introduction of the worker safety legislation into REACH were further reiterated at the HCF.
This is what we at Ecobio found to be the most relevant discussion points at HCF 2021. Hope you enjoyed the reading. Next HCF will be held in March 2022 and thereafter every second year. You can find the CSS here and the CSS action plan here.
Do you need help with chemical management?
Our experienced chemical consultants will assist you in meeting your chemical requirements. Furthermore, our Ecobio Manager SaaS-service will help you manage your chemicals and ensure compliance with global regulations. Interested? Contact us today!
You can contact us through email at info@ecobio.fi or by phone +358 20 756 9450.
You might be interested in our chemical management webinar on Thursday 6.5.2021
Welcome to our webinar regarding the digital future of chemical management on Thursday the 6th of May 2021. In our webinar our we summarize chemical risks for companies as well as their management in the workplace. Our experts present the most common challenges and digital solutions regarding complying with chemical laws. Additionally we go trough managing the use of chemicals and assessing the risk of chemical exposure. The webinar is held in both Finnish, Swedish, and Norwegian.
Read more and register using the links below:
You can find all of our upcoming and recorded webinars from our webinar library here!
Text: Mari Eskola, Dr, Senior Consultant
Picture: Shutterstock




 Anne Kallioinen
Anne Kallioinen